Abstract
Introduction
Hereditary thrombotic thrombocytopenic purpura (hTTP) is an ultra-rare thrombotic microangiopathy caused by autosomal recessively inherited severe ADAMTS13 deficiency. In respect to organ damage, we recently reported a heterogeneous clinical course with some patients having various degrees of organ damage while others are almost asymptomatic*. The long-term consequences of hTTP are still not fully known.
Methods
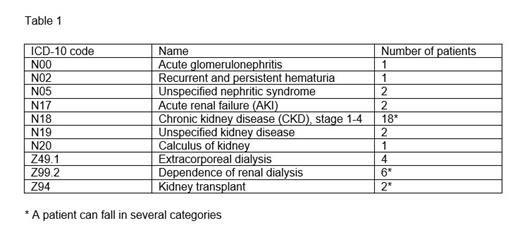
We analyzed the prevalence and development of renal disease in confirmed hTTP patients in the International Hereditary TTP Registry until July 2021. We studied the onset of kidney disease, the presence of additional co-morbidities and the possibility of a specific genotype-phenotype correlation. We classified different renal diseases of our hTTP patients, according to the ICD-10 code (version 2016) as described in Table 1.
Results
Thirty-five (21%) of 163 confirmed hTTP patients of the International TTP Registry with a mean age at enrolment of 38.2 years had kidney disease. 80% were Caucasian, 8.5% Asian and 11.5% indicated other ethnicities.
According to the ICD-10 code classification, two patients had acute kidney disease (AKI, ICD code N17) not specified during acute TTP episodes. Among 18 patients with general CKD, we could identified three patients with chronic kidney disease (CKD, ICD code N18) stages 1-2; four with CKD stage 3, and four patient with CKD stage 5. In two patients, kidney disease was unspecified (ICD code N19). Five patients had diagnoses of certain specific renal diseases, such as acute glomerulonephritis (ICD code N00), unspecified nephritis syndrome (ICD code N05), recurrent and persistent hematuria (ICD code N02), and calculus of the kidney (ICD code N20) (Table 1). Seven patients developed end-stage renal disease requiring renal replacement therapy (ICD code Z99.2), which was temporarily needed in four additional patients (Z49.2). Two patients underwent kidney transplantation (ICD code Z94) (Table 1).
The 163 hTTP patients experienced 625 acute disease episodes, of which 108 (17%) were associated with acute renal failure. 21/163 (13%) acute episodes were treated with hemodialysis. After experiencing several hTTP episodes with acute renal failure, 15 patients developed CKD at different stages.
26/35 (74%) hTTP patients with kidney disease presented also other co-morbidities: hypertension in 14 patients, 10 and 18 patients had suffered from transient ischemic attacks and/or strokes, and six patients had suffered from a myocardial infarction. We observed no apparent correlation between onset of CKD and presence of co-morbidities.
Regular plasma prophylaxis was introduced in 26/35 hTTP patients with kidney disease; in the majority of them (64%) plasma treatment was started due to CKD or after CKD diagnosis.
Of the 35 patients with renal disease, 15 were homozygous and 20 compound heterozygous ADAMTS13 mutation carriers. The most known ADAMTS13 mutation c.4143_4144dupA counts 33% of the whole hTTP patients in our database (54/163 patients). It was the most represented mutations in our cohort of hTTP patients with renal disease (60%, 21/35): 11 homozygous and 10 compound heterozygous carriers. Nevertheless, we did not observe a specific genotype-phenotype correlation.
Conclusions
Our analysis of the International Hereditary TTP Registry cohort (at present 163 patients) showed that the majority of patients had a diagnosis of hTTP before a diagnosis of kidney disease, especially CKD. We could not identify a correlation with the onset of additional co-morbidities or with the genotype; nevertheless we cannot exclude that it may be present. Considering that 43% (15/35) patients developed CKD after several acute episodes with acute renal failure, a closer and attentive care for those patients not yet developing CKD to avoid or delay the onset is highly recommended. Hence, to evaluate the contribution of acute hTTP episodes and of other co-morbidities to the development of kidney disease requires further in depth analyses. More frequent and long-term follow-up visits are needed to better understand the long-term outcome and development of kidney disease in hTTP patients.
* Tarasco et al, Blood 2021
Matsumoto: Sanofi: Consultancy; Takeda: Consultancy; Alexion Pharma: Consultancy; Asahi Kasei Pharma: Research Funding; Chugai Pharmaceutical: Research Funding; Alfesa Pharma: Patents & Royalties: ELISA for measuring ADAMTS13 activity. Lämmle: Takeda: Membership on an entity's Board of Directors or advisory committees; Ablynx: Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Baxter: Other: Travel Support, Speakers Bureau; Alexion: Other: Travel Support, Speakers Bureau; Siemens: Other: Travel Support, Speakers Bureau; Bayer: Other: Travel Support, Speakers Bureau; Roche: Other: Travel Support, Speakers Bureau; Sanofi: Other: Travel Support, Speakers Bureau. Kremer Hovinga Strebel: Baxalta US Inc: Other: grant; Shire: Consultancy, Speakers Bureau; Ablynx: Consultancy, Speakers Bureau; Federal Office of Public Health: Consultancy; Insel Gruppe AG: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal